Cervical cancer is caused by a persistent infection (>2 years) with high-risk human papillomavirus (hrHPV). This means infection with the strains of HPV that are most likely to cause cancer – known as oncogenic strains.
Cervical cancer is preceded by pre-cancerous changes in the cervix called cervical intraepithelial neoplasia (CIN). This is normally asymptomatic and is normally only picked up during screening.
CIN is defined by dyskaryosis – mutations in the squamous cells in the transformation zone of the cervix. Mutations are much more likely to happen here at the squamocolumnar junction as the cells are already ‘transforming’ from squamous to columnar known as metaplasia.
Risk Factors and Prevention
- HPV 16 and 18
- Early first sexual experience
- Multiple partners
- Smoking
- Immunosuppression with HIV
- COCP – N.B – this may be more likely an incidental factor linked with use of non-barrier contraception and multiple sexual partners rather than hormonal effects of the pill.
Nationally all school children are offered the HPV vaccine (Gardasil), normally between the ages of 11-13. This protects against them contracting HPV strains 6,11 (to prevent genital warts) and 16 and 18 (to prevent cervical neoplasia and cervical, vulval, vaginal and anal cancer).
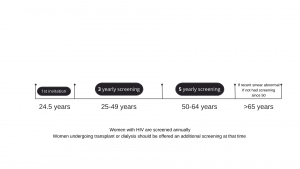
Cervical Screening Programme
Cervical screening is available to women and people with a cervix aged 25-64. The programme has been extremely successful, with the number of women dying from cervical cancer being halved since its introduction. There still remain some barriers to screening, particularly in younger women such as embarrassment, fear and inconvenience.
The Screening Process
A speculum is inserted and a brush is rotated against the transformation zone of the cervix. The brush head is then sent off to the laboratory for testing:
- HPV screening – testing for HPV first is more sensitive and accurate.
- LBC (liquid based cytology) looks for dyskaryosis.
We used to use the Papanicolau (Pap) smear test – which was just another way of looking at the cells. This has been replaced by liquid based cytology testing, only if the sample is HPV positive.
Urinary HPV testing is planned for the future to try and reduce common barriers to a ‘smear’ test. This would involve a self-collected first stream urine sample alongside a vaginal sample. Studies have been promising in showing high rates of detection of HPV in a way that is much more acceptable to women.
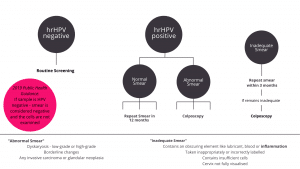
Understanding the Results
Management
Colposcopy is required to diagnose and stage CIN. This involves visualisation of the cervix; the clinician will then stain the cervix with Acetic Acid. This makes abnormal areas turn white, known as Acetowhitening. This is due to acetic acid’s role in coagulating abnormal proteins, therefore the more abnormal proteins present, the more abnormal an area, the whiter it becomes.
Secondly Iodine stain is used to look for abnormal cells. Remember how CIN normally occurs in the squamous cells at the squamocolumnar junction. Normal squamous tissue contains glycogen whereas columnar cells do not. As Iodine is glycophillic, application will cause uptake of the stain in the normal tissue (where there is no glycogen) but the CIN or cancer will remain unstained (a saffron-yellow)
Finally, a biopsy is taken for histological diagnosis
Only high-grade dysplasia – CIN II or III should be treated. This can occur at the same time as colposcopy – ‘see and treat’ or at an additional time. The most common form of treatment is large loop excision of the transformation zone – a LLETZ biopsy.
Other options include cryotherapy, laser or cold coagulation to destroy or excise the abnormal area. Patients should be given local anaesthetic before the area is removed. If the abnormal area extends into the cervical canal a Cone Biopsy is indicated.
Recent research has shown there is no reduction in fertility with procedures for pre-cancerous changes of the cervix but there is increases in rates of miscarriage and pre-term delivery.
Important to Note
- Transgender men who have retained their cervix should be included in the NHSSCP programme unless they have made the informed decision to opt-out
- Non-binary individuals must be invited by their individual GP to participate as their gender is not currently captured by the NHSCSP.
- Screening should be delayed for pregnant individuals until 12 weeks post-partum